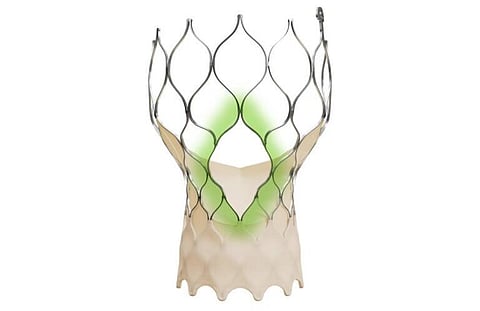

Medtronic has announced the launch of its Evolut FX+ transcatheter aortic valve implant (TAVI) in India, following approval from the Central Drugs Standard Control Organization (CDSCO) in June. The system had earlier received FDA approval and the CE mark.
The Evolut FX+ introduces a redesigned diamond-shaped frame with three enlarged coronary access windows—four times larger than in earlier Evolut versions. This improvement provides physicians with greater catheter maneuverability and easier access to coronary arteries across diverse anatomies, while maintaining the valve’s radial strength, hemodynamics, and overall performance.
Designed for patients with severe aortic stenosis, especially those who may require future coronary interventions, the Evolut FX+ represents a significant advancement in TAVI therapy.
“The launch of Evolut FX+ in India marks a meaningful step forward in transcatheter valve therapy. With its enhanced coronary access and proven hemodynamic performance, FX+ empowers physicians to deliver confident care—not just during the initial procedure, but throughout the patient’s lifetime,” said Prateek Tiwari, senior director, Neuroscience & Speciality Therapies, Medtronic.
He added, “By bringing this innovation to India, we are advancing Medtronic’s mission to alleviate pain, restore health, and extend life, while expanding access to future-ready solutions for structural heart disease.”