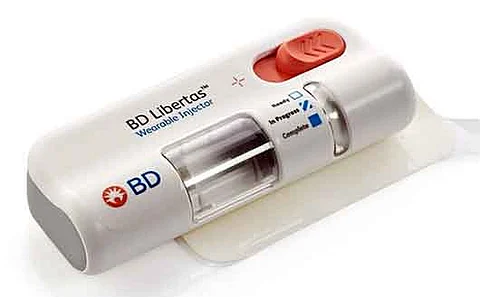

BD (Becton, Dickinson and Company) , a global leader in medical technology, has announced the initiation of the first pharmaceutical company-sponsored clinical trial utilizing the BD Libertas™ Wearable Injector, a novel device designed for the subcutaneous administration of complex biologics.
This milestone builds on the success of more than 50 pre-clinical and clinical studies conducted by BD, including a recent device-specific clinical study in which 100% of participants indicated they would be likely to use the BD Libertas™ Wearable Injector if prescribed.
The trial marks a major step forward in the evolution of drug-device combination products, enabling a shift from clinic-based infusions to more flexible treatment options—including at-home self-injection. This could significantly enhance patient convenience, adherence, and access to care.
“This trial exemplifies BD’s commitment to supporting pharmaceutical partners in advancing large-volume injection science,” said Patrick Jeukenne, Worldwide President of BD Pharmaceutical Systems. “By making therapies more accessible and user-friendly, we are helping drive the future of biologic drug delivery.”
The BD Libertas™ Wearable Injector is a prefilled, ready-to-use, mechanical drug delivery system specifically engineered for high-viscosity biologics. As the biologics market is projected to surpass $670 billion by 2030iv, this platform offers pharmaceutical developers a versatile and patient-centric solution.
Key features of the BD Libertas™ Wearable Injector include:
Support for high-viscosity formulations (up to 50 centipoise), expanding compatibility across a wide range of subcutaneous therapies
Multiple volume options (2–5 mL and 5–10 mL), accommodating diverse therapeutic needs
User-friendly design with a simple “peel, stick, and click” application—no filling or assembly required by the patient.
In addition, BD’s collaboration with Contract Manufacturing Organizations (CMOs) for fill-finish and final assembly validation positions the company to support pharma partners from early development through to commercial-scale production.
To learn more about how the BD Libertas™ Wearable Injector is transforming the delivery of biologic therapies.