
Hologic, Inc. has announced that its Genius™ Digital Diagnostics System has received expanded CE marking in the European Union, now approved for imaging and reviewing both cell and tissue specimens. Previously cleared only for cellular analysis—especially in cervical cancer screening—the system can now perform whole slide imaging across a broader array of patient sample types.
With full-slide digital imaging capability, the platform can support workflows such as identifying precancerous or cancerous cervical cells during screening and reviewing cervical tissue biopsies when abnormalities are detected. In breast health, the system enables digitization and remote review of breast biopsy tissue, an essential step in diagnosing or ruling out malignancy. The expanded functionality extends to many other specimen types, allowing laboratories to consolidate processes and increase flexibility.
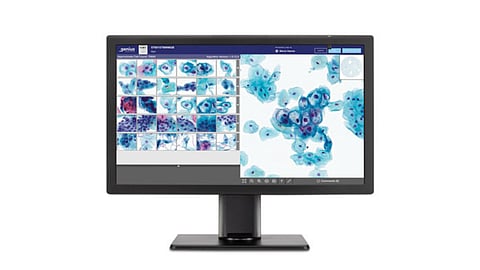
Most diagnostic labs currently depend on separate systems for different specimen categories, which can lead to inefficiencies, higher operational costs, and longer turnaround times. Hologic’s system uses advanced volumetric imaging technology to capture high-quality images of both cells and tissues, storing and distributing them through a single digital platform to streamline workflows.
The expanded CE marking highlights the potential for digital pathology to enhance diagnostic accuracy, efficiency, and overall patient care. By enabling more specimen types to be processed on one platform, the system supports modernized, integrated laboratory operations.
Using volumetric imaging, each glass slide is converted into a high-resolution digital image by capturing 14 layers of the specimen and merging them into a single two-dimensional view. Completed cases are then sent to a secure image management server for storage and remote or local review. The expansion also introduces upgraded software features, including remote support, LIS readiness, and enhanced review tools.
Hologic’s digital pathology technologies have been CE marked under the In Vitro Diagnostic Regulation, recognized across the EU and other global markets. Commercial availability will be communicated country by country. Whole slide imaging capability is not currently available in the United States.
Also Read